Which of the Following Describes the Bohr Atomic Model
Hybrid orbitals do not exist in isolated atoms. The optimum size of a sphere of very.
Bohr Model Questions and Answers.

. Chemistry End of Chapter Exercises. Examples include the discovery of fire extracting metals from ores making pottery and glazes fermenting beer and wine extracting chemicals from plants for medicine and. A set of hybrid orbitals is generated by combining atomic.
See the atomic model of James. By 1000 BC civilizations used technologies that would eventually form the basis of the various branches of chemistry. 0 - Bohr Model radius of a 0 2s 2 peaks Maximum at r 5 a 0 - Bohr Model radius of 4 a 0 3s 3 peaks Maximum at r 13 a0 - Bohr Model radius of 9 a 0 These maximum correspond to the distance from the nucleus at which the electron has the highest probability of being found ie.
Motion of electrons 26. What are the main points of Daltons atomic theory. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table.
Fluorine is a pale yellow gas that reacts with most substancesThe free element melts at 220 C and boils at 188 CFinely divided metals burn in fluorine with a bright flameNineteen grams of fluorine will react with 10 gram of hydrogen. Describe energy levels of electrons. Sign up for new.
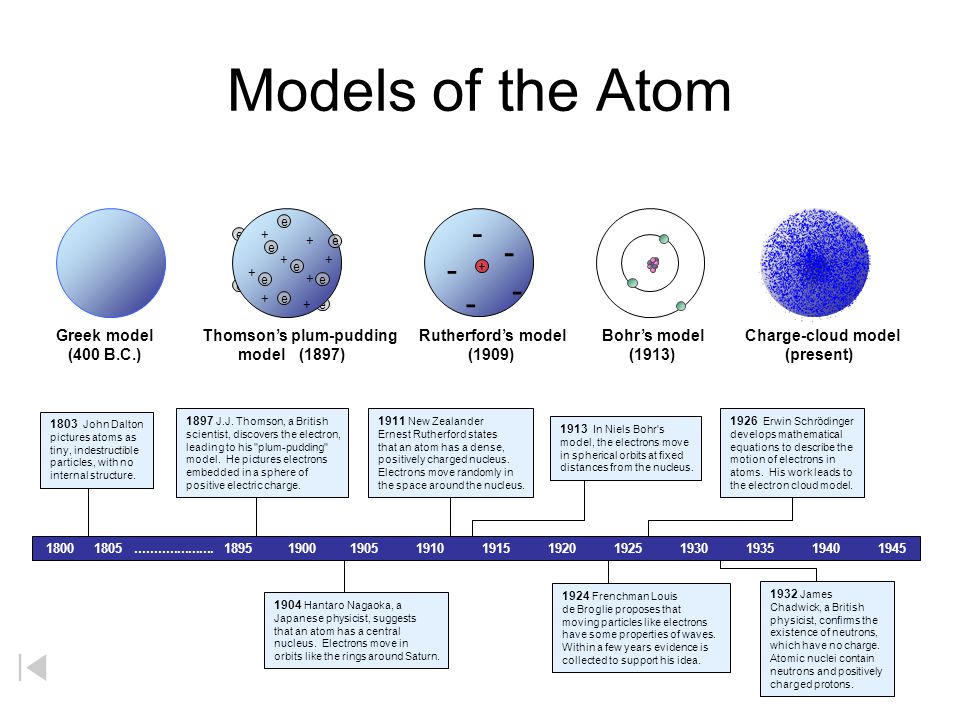
Electrical charges of particles d. The Bohr model of the atom was proposed by Neil Bohr in 1915. The history of chemistry represents a time span from ancient history to the present.
It came into existence with the modification of Rutherfords model of an atom. Atomic models have been improved over the years. The following ideas are important in understanding hybridization.
They are formed only in covalently bonded atoms. Solving for the wavelength of this light gives a value of 4863 nm which agrees with the. Classify the six underlined properties in the following paragraph as chemical or physical.
According to the Bohr model the wavelength of the light emitted by a hydrogen atom when the electron falls from a high energy n 4 orbit into a lower energy n 2 orbitSubstituting the appropriate values of R H n 1 and n 2 into the equation shown above gives the following result. Look at the diagram. Get help with your Bohr model homework.
Journal of Physics B. The chemical symbol for Hydrogen is H. Schrödinger equation 5 and for N particles the difference is that the wavefunction is in 3N-dimensional configuration space the space of all possible particle positions16 This last equation is in a very high dimension so that the solutions are not easy to visualize.
Bohrs changes in Rutherfords model of the atom involved the a. Then read the questions that follow. Structure of the nucleus b.
Learn the theory of James Chadwick his experiment and his contribution to atomic theory including his discovery. Access the answers to hundreds of Bohr model questions that are explained in. Number of particles c.
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. Its monatomic form H is the most abundant chemical substance in the Universe constituting. An electron shell is the set of allowed states that share the same principal quantum number n the number before the letter in the orbital label that electrons.
Arrange the following atomic models in the order of their chronological order i Rutherfords atomic model ii Thomsons atomic model iii Bohrs atomic model a i ii and iii b ii iii and i c ii i and iii d iii ii and i - Get the answer to this question and access a vast question bank that is tailored. Rutherfords model introduced the nuclear model of an atom in which he explained that a nucleus which is positively charged is surrounded by negatively charged electrons. Submit an article opens in new tab Track my article opens in new tab.
Atomic Molecular and Optical Physics covers the study of atoms ions molecules and clusters and their structure and interactions with particles photons or fields. Hybrid orbitals have shapes and orientations that are very different from those of the atomic orbitals in isolated atoms. Electron configuration was first conceived under the Bohr model of the atom and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.

Bohr Atomic Model Bohr Model Of An Atom Bohrs Model Of The Hydrogen Atom Atom Chemistry Lessons Atom Model

Atomic Models And Their Innovations Atomicmodel Atomicmodels Daltonmodel Thomsonmodel Rutherfordmodel Bohrmod Chemistry Lessons Atom Chemistry Classroom
Comments
Post a Comment